|
LSK Global PS’s Clinical Study Contracts Top1,000, Solidifying Its Position as Leading Korean CRO
- Asof March 2018, LSK Global PS has conducted a total of 1,013 clinical trials --116 global and 897 local clinical trials -- for pharmaceutical companies. - Asa Korea-based CRO dedicated to providing one-stop full service across all theclinical trial areas, the company has accelerated advance in CRO services sinceits foundation 18 years ago.
[April 3, 2017] Korea-based leading contract research organization LSKGlobal Pharma Services Co., Ltd. announced today that the number of clinicalstudies conducted by the company has topped 1,000 as of March 2018. This featcomes as the company celebrates its 18th anniversary. LSK Global PS was foundedin March 2000
More than 1,000 local and global clinical trialsconducted since foundation of LSK Global PS LSK Global PS has conducted atotal 1,013 of clinical trials including 116 global and 897 local clinicaltrials since its foundation (March 2000 ? March 2018). Behind the impressivenumber are the company’s know-how and capabilities in providing high-qualityefficient clinical trial services which meet rigorous global standards.
The companyhas expanded its experience with study type-specific and therapeuticarea-specific clinical studies. By study type, it has provided integratedservice across all areas, including 575 registration trials from phase I to IIIclinical trials, 41 investigator-led studies and 237 post-approval clinicalstudies such as phase IV clinical trials, post-marking surveillence andobservational studies. (see the below graph.) By therapeutic area, it hasconducted 181 trials in cardiovascular disease, 173 in oncology, 99 inendocrinology and 57 in neurology. 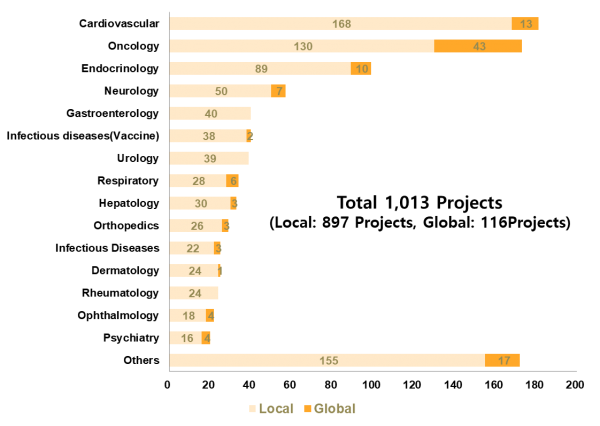
Graph 1. Number of clinicaltrials conducted by LSK Global PS by therapeutic area (as of March 2018)
LSK Global PS has conducted atotal of 83 clinical trials for investigational new drug applications (INDs).By study type, phase III registration trials accounted for the largestproportion of studies, and by therapeutic area, oncology represented thelargest proportion. In the early years of the company, it mainly conducted INDclinical studies for global pharmaceutical companies. In recent years, however,IND clinical trials have been conducted increasingly for local pharmaceuticalcompanies and bio ventures. Graph 2& 3. Number of IND clinical studies conducted by LSK Global PS by studytype / therapeutic area (as of March 2018) In addition, the company’sexperience in study rescue deserves attention. LSK Global PS has expanded itsexperience and capabilities in conducting high-quality clinical trials whichcan help pharmaceutical companies to solve problems and produce favorableresults at various stages of clinical trials. It has rescued a total of 11clinical trials across various areas including Quality Control & Audit,Data Management, Statistics and Clinical Operation. Currently, it is providingdata management, statistics, result reporting and drug monitoring services as apart of a study rescue contract with a major global CRO. The quality of LSK Global PS’sclinical trial development and operation services has been demonstrated insystem audits targeting a number of global CROs and pharmaceutical companies aswell as the Ministry of Food and Drug Safety’s site inspections. In addition,the company outperformed a leading global CRO and became the first Korean CROto win a contract with a global pharmaceutical company for a phase I clinicaltrial of a first-in-human oncology drug. One-stopfull-service CRO encompasses all the major areas of clinical trials LSK GlobalPS has established its position as a one-stop, full-service CRO encompassingall of the major areas of clinical research, i.e. registration trials fromphase I to III, investigator-sponsored clinical studies, post-registrationstudies such as phase IV studies, PMS studies and observational studies as wellas consulting services for new drug development. LSK Global PS provides allof areas of services related to clinical trials such as Medical Writing &Research, Product Development, Regulatory Affairs, Study Start Up, ClinicalOperation,Project Management, DataManagement, Biostatistics, Quality Assurance, Epidemiological Research,Pharmacovigilance, and Training. The company has maintainedtransparency in sharing clinical data, providing detailed information about databy therapeutic area, clinical stage and service type at its website. |